- ...
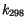 4.1
4.1
- Units for first order reactions are s
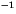 ,
for the second-order gas reactions are molecules
,
for the second-order gas reactions are molecules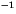 cm
cm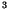 s
s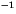 ,
for the third-order gas reactions molecules
,
for the third-order gas reactions molecules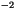 cm
cm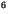 s
s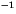 , and
for the second-order aqueous reactions are M
, and
for the second-order aqueous reactions are M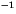 s
s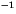 . Units for
solubility constants are M atm
. Units for
solubility constants are M atm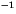 , and units for
dissociation constants are M. Reaction rates are of the form
, and units for
dissociation constants are M. Reaction rates are of the form
![$ k = k_{298} \hbox{exp}[-{E \over R} ({1 \over T} - {1 \over 298})]$](img2813.gif) unless otherwise noted.
unless otherwise noted.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... &Reference4.2
- NASA97, Demore et al. [49]; Y90,
Yin et al. [195,194]; HC85, Hoffmann and Calvert [72];
LK86, Lind and Kok [112]; NBS65, National Bureau of Standards [131]; and M82,
Maahs [117].
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... MSA4.3
- Here
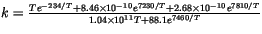 .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... 4.4
- Here
![$ k = {{k_{298}\hbox{exp}[-{E \over R}
({1 \over T} - {1 \over 298})] [H^+]} \over {1 + 13[H^+]}}$](img2815.gif) .
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
- ... time.6.1
- Mid-month concentrations are input
and then interpolated to daily values. The input data are constructed
to correctly recover the observed monthly means value using the
method of Taylor et al. [170]
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.